Impacts of Chemicals
Highly alkaline solutions with high temperature has highest corrosion
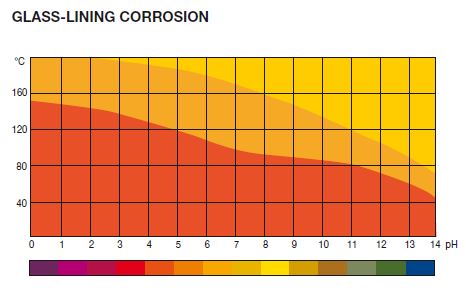
We can see graph Glass Lining Corrosion graph Temperature vs pH
Excellent resistance to corrosion
Variable effect depends on actual process
HCl
- If HCl is using in liquid phase in glasslined reactor it will erode 0.01mm / year.
- HCl in Vapour phase corrode 0.04 mm/year.
NaOH
- NaOH is erode 0.21 mm/year.
Water
- Water erodes 0.008 mm/year in liquid phase.
- Water vapour erodes 0.013 mm/year.
Erosion is increasing with increase in batch volume and fluid velocity in reactor.
We can see in below graph that corrosion is minimum up to 150℃.
Corrosion is increasing with in increasing temperature and alkaline pH.
We can see general Acid base vs Volume below






Post a Comment